Этнографический салон "Sary Azman" представляет собой уникальное культурное пространство, где каждый может познакомиться с
Детективное агентство "След", как и многие современные детективные агентства, предлагает услуги, которые могут быть
Юбки бывают разных длин, фасонов и материалов, каждый из которых подходит для различных случаев
В настоящее время все больше женщин предпочитают покупать одежду онлайн, в интернет-магазинах, и это
В этой статье мы поговорим о привлекательности и особенностях колец с рубином, а также
Для фотографов, как начинающих, так и профессионалов, аренда фотостудии с полным набором оборудования часто
Основная задача таких клиник – предоставить пациентам комплексную помощь и поддержку в процессе лечения
Такой формат аренды дает вам дополнительное время на возврат костюма, делая процесс аренды еще
Женская одежда в этно-стиле приносит в мир моды уникальный дух и национальный колорит, объединяя
Пицца – это кулинарное искусство, началом которого является правильно приготовленное тесто. Основа хорошего теста
Индивидуальные мерки: Костюм должен быть изготовлен с учетом индивидуальных мерок тела, таких как обхват
Маяк Анива расположен на самой восточной точке острова Сахалин, в Южно-Курильском районе Российской Федерации.
Обувь из материала ЭВА имеет ряд преимуществ, которые делают ее привлекательным выбором для различных
Покупка новой обуви всегда вызывает радость, но также и новые заботы о сохранении ее
Мужские костюмы Миллионер – это коллекция стильных и элегантных костюмов, производимых из высококачественных тканей
Рабочая спецодежда включает в себя комплекты, костюмы, куртки, брюки, комбинезоны, халаты и другие предметы
Если вы хотите заказать пошив футболок с капюшоном, то вам нужно найти производителя, который
Лечение наркозависимости — это сложный процесс, связанный с отказом от употребления наркотиков и восстановлением
Материал – самый важный критерий, который необходимо учитывать при покупке постельного белья. Он указывает
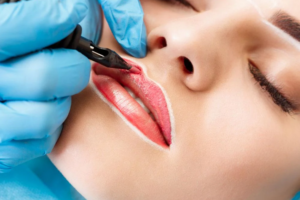
Если вы думаете о нанесении перманентного макияжа , то стоит потратить некоторое время, чтобы
Существуют также нестандартные варианты с емкостью 600 мл и выше. Необходимо учитывать свои привычки
За счет разделения наполнитель не собирается в кучу и не слеживается. Трехкамерные изделия чаще
Кроме растворителей, при очищении одежды используются и другие химические материалы, применяемые для обезжиривания, зачистки
Чаще всего производят такие украшения из следующих материалов: золото, серебро или платина. Чтобы выбрать
Цветотипа Зима, фото, примерами | Описание подтипов: цветотип яркая зима, цветотип светлая зима, цветотип
Цвета подходящие цветотипу зима весьма разнообразны. И тем не менее необходимо знать. какие оттенки
Цветотип Весна: характеристика, какие цвета подходят в макияже и одежде, цветотип Весна фото примеры.